How to Calculate Partial Pressure
Of the dry air and of the water vapor. Potential_temperature pressure temperature.
Mixtures Of Gases Partial Pressures
Definition of partial pressure and using Daltons law of partial pressures.
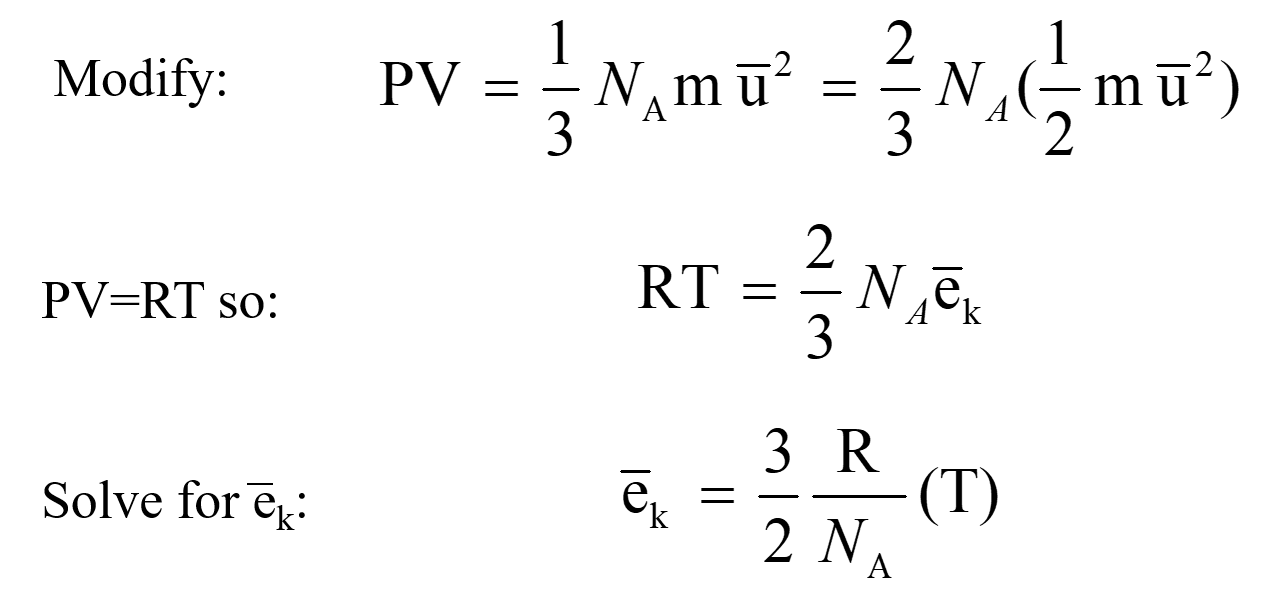
. The flow meter will be set between 8 and 15 liters. To calculate partial pressure start by applying the equation k PV to treat the gas as an ideal gas according to Boyles law. The cubic feet per minute CFM of a gas describes its volumetric flow rate through a pipe or vent.
What Is A Partial Pressure. You have to divide the pressure exerted by the air into two partial pressures. Vapor pressure is measured in the standard units of pressureThe International System of Units SI recognizes pressure as a derived unit with the dimension of force per area and designates the pascal Pa as its standard unit.
Then convert the equation into Kelvin if it isnt already by adding 273 to the temperature in Celsius. At 20C 68F or 29315K at standard atmospheric pressure. When adhesive forces are greater than cohesive forces the liquid is said to be wetting When cohesive forces exceed adhesive forces the.
Calculate the pressure drop of a liquid having flow of 10 m per sec. Combining these two values gives you the desired parameter. Gas mixtures and partial pressures.
At a temperature of 300K 30 litres of gas A kept under pressure of 1 atm and 15 litres of gas B kept under pressure of 2 atm is transferred into an empty 10L container. In Imperial or US customary measurement system the density is equal to 624 pound per cubic foot lbft³ or 058 ounce per cubic inch ozinch³. Calculate the saturation vapor pressure at given temperature T using the formula p₁ 61078 1075T T 2373 where T is measured in degrees Celsius.
One pascal is one newton per square meter Nm 2 or kgm 1 s 2. While the ideal gas law can still offer an approximation under these conditions it becomes less accurate when molecules are close together and excited. Partial pressure is the pressure that a certain gas mixture component would have in the same temperature and volume if it were on its own.
Observe that a partial rebreather oxygen mask will deliver 35 to 60 percent of oxygen. The sum of partial pressures of all components is equal to the total pressure. Daltons Law of Partial Pressure.
Density of water is equal to 1 000 kgm³. If youre seeing this message. To use the formula for a real gas it must be at low pressure and low temperature.
In Imperial or US customary measurement system the density is equal to 70805793 pound per cubic foot lbft³ or 655609 ounce per cubic inch ozinch³. The inner diameter is 05 m and the friction factor is 03. Calculate water vapor partial pressure.
After youve done that begin finding the partial pressure of each gas by using the formula P nRTV. An online partial pressure calculator is properly designed to calculate partial pressure volume temperature and amount of moles of each individual gas enclosed in a container. From the ideal gas equation PV nRT.
The Bernoulli equation states that velocity is determined by calculating difference in pressure between two points multiplying by 2 dividing by the density of water and then taking the square root. Density of lead is equal to 11 342 kgm³. If the pressure is known in pounds per square inch or psi at two locations along the pipe then the Bernoulli equation can be used to determine the velocity of the water.
Lead weighs 11342 gram per cubic centimeter or 11 342 kilogram per cubic meter ie. At 25C 77F or 29815K at standard atmospheric pressure. The flow meter should never set below 8 liters.
Water weighs 1 gram per cubic centimeter or 1 000 kilogram per cubic meter ie. Daltons law of partial. To picture this speed calculate the.
Before you move further you need to understand important gas laws which are explained below. Calculate pressure-weighted mean of an arbitrary variable through a layer. In the context of chemistry.
They are commonly used in thermodynamics cardiovascular physiology and respiratory physiology. A pressurevolume diagram or PV diagram or volumepressure loop is used to describe corresponding changes in volume and pressure in a system. Gravity R - radius of the planet or object on which you calculate surf.
The volumetric flow is a good measure of how much gas goes through the system but it isnt the clearest way of picturing how quickly it moves. The liter flow is based on your observation. Once again the derivative gives the slope of the tangent line shown on the right in Figure 1023Thinking of the derivative as an instantaneous rate of change we expect that the range of the projectile increases by 5095 feet for every radian we increase the launch angle y if we keep the initial speed of the projectile constant at 150 feet per second.
Using the ideal gas law to calculate a change in volume. G G M R 2. Where G - Gravitational constant 66710-11 Newton-meter 2 kg 2 M - mass of the planet or object on which you calculate surf.
Vertical_velocity omega pressure temperature Calculate w from omega assuming hydrostatic conditions. Pressure drop is created when a difference in pressure arises between the two points of the. Capillary pressure results from interactions of forces acting within and between fluids and their bounding solids.
How to Calculate CFM to MPH. Calculate the total pressure inside the container and the partial pressures of gas A and gas B Assume that A and B are ideal gases. Experimental measurement of vapor pressure is a.
You can calculate the partial pressure by multiplying the mole fraction of the gas component by the total pressure of the gas. Increasing pressure or temperature raises the kinetic energy of the gas and forces the molecules to interact. These include both cohesive forces surface and interfacial tension and adhesive liquid-solid forces.
PV diagrams originally called indicator diagrams were developed in the 18th century as tools for understanding the.
Molecular Formulas And Nomenclature

Gas Mixtures And Partial Pressures Video Khan Academy
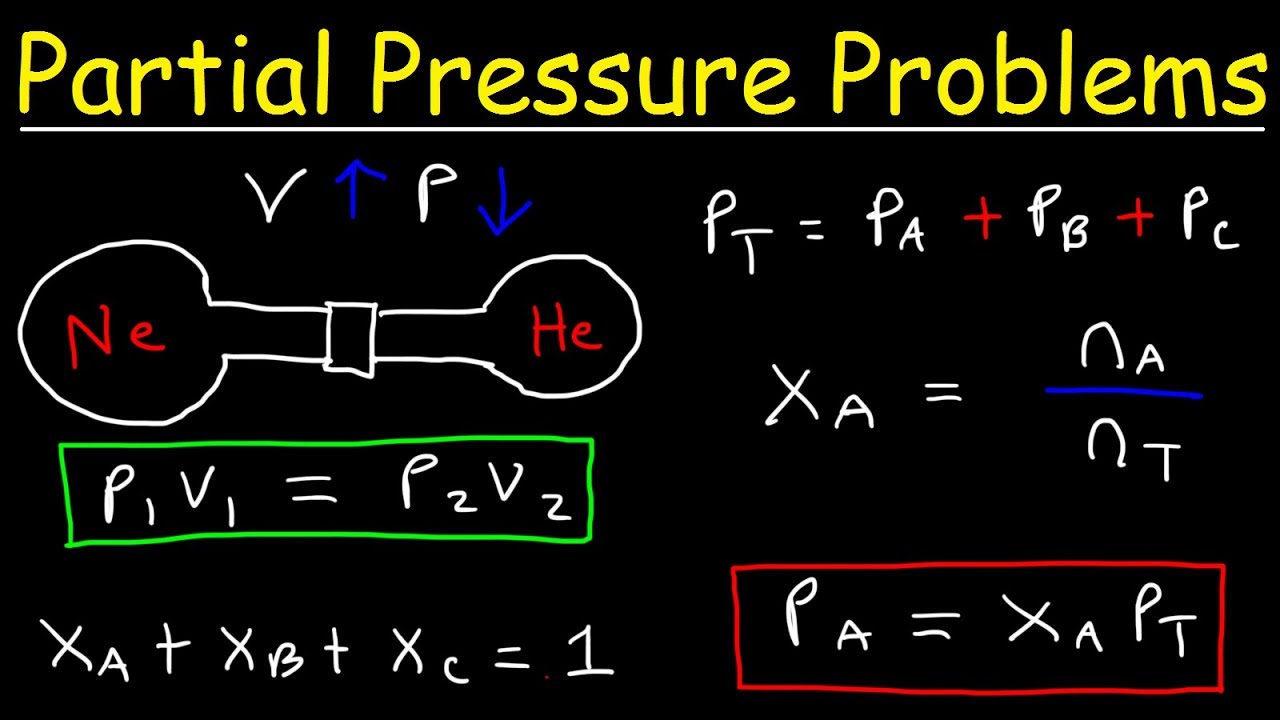
Dalton S Law Of Partial Pressure Problems Mole Fraction Chemistry Gas Laws Youtube

Partial Pressures Of Gases And Mole Fractions Chemistry Tutorial Youtube
0 Response to "How to Calculate Partial Pressure"
Post a Comment